Assisting with breathing using a ventilator to deliver oxygen. Theres currently no cure for SARS but research to find a vaccine is ongoing.
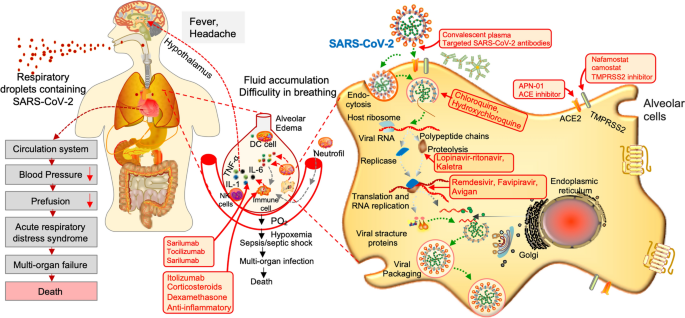 Pathophysiology And Treatment Strategies For Covid 19 Journal Of Translational Medicine Full Text
Pathophysiology And Treatment Strategies For Covid 19 Journal Of Translational Medicine Full Text
The main symptoms are pneumonia and fever.

Sars virus treatment. V ratio and mixed. Over the next few months the illness spread to more than two dozen countries in North America South America Europe and Asia before the SARS global outbreak of 2003 was contained. SARS became a much-talked about disease in 2003 when over 8000 cases and 774 deaths occurred worldwide.
An infected person may also need a great deal of supportive treatment including artificial ventilation to help with breathing. Chronic infection with SARS-CoV-2 leads to the emergence of viral variants that show reduced susceptibility to neutralizing antibodies in an immunosuppressed individual treated with convalescent. An inactivated whole virus was used in ferrets nonhuman primates and.
The SARS outbreak of 2002-2003 presented clinicians with a new life-threatening disease for which they had no experience in treating and no research on the effectiveness of treatment options. Methylene blue MB was added to 200 mL of plasma virus mixture and mixed to a final concentration of 1 μM a 2 μM b or 4 cd was set only virus. A person suspected of having SARS should be admitted to hospital immediately and kept in isolation under close observation.
What about a SARS vaccine. 3 The mechanism of action of ribavirin has been studied for decades and is still under active debate. ANTIVIRAL AGENTS Ribavirin.
Vaccine studies for SARS-CoV-1 were started and tested in animal models. To 180 mL of healthy human plasma 20 mL of indicator virus was added at a 9. Treatment is mainly supportive and may include.
VERO-E6 cells infected by SARS-CoV-2 across different treatment groups 100. However some medicines have been used including steroids and antiviral medicines. The specific treatment for SARS and MERS is still being researched.
SARS-CoV the virus that caused SARS which was first identified in 2003. Treatment of SARS-CoV-2 Virus Disease COVID-19 in Humans With Hemopurifier Device The safety and scientific validity of this study is the responsibility of the study sponsor and investigators. The World Health Organization WHO expert panel on SARS treatment requested a systematic review and comprehensive summary of treatments used for SARS-infected patients in order to guide future.
Antiviral medications and steroids are sometimes given to reduce lung swelling but. There is no confirmed treatment that works for every person who has SARS. It is a nucleoside analogue that has activity against a number of DNA and RNA viruses in vitro.
MERS-CoV the virus that caused Middle East respiratory syndrome MERS which was first identified in 2012. Severe acute respiratory syndrome SARS is caused by a virus. SARS was first reported in Asia in February 2003.
Antibiotic drugs dont work against viruses and antiviral drugs havent shown much benefit. Ribavirin is used extensively for the treatment of SARS and was given to over 90 of patients in Hong Kong. 4 In early March 2003 before the isolation of the SARS-CoV.
Listing a study does not mean it has been evaluated by the US. Severe acute respiratory syndrome SARS is a viral respiratory illness caused by a coronavirus called SARS-associated coronavirus SARS-CoV. The virus is usually passed on when people sneeze or cough.
Interferon IFN is not recommended as standard therapy in SARS. The rationale for development of vaccines against SARS-CoV is to provide a means of control in the event a new SARS-CoV epidemic occurs or there is a deliberate release of the virus. Explore Mayo Clinic studies testing new treatments interventions and tests as a means to prevent detect treat or manage this condition.
Inactivated SARS-CoV vaccine has been shown to induce neutralizing antibodies that block binding of the virus to its receptor ACE2. Despite a concerted global effort scientists have yet to find an effective treatment for SARS. Kaletra 400 mg ritonavir and 100 mg lopinavir a protease inhibitor used in the treatment of human immunodeficiency virus infection may be considered for early treatment of SARS patients preferably in a randomised double-blind placebo-controlled clinical trial setting.