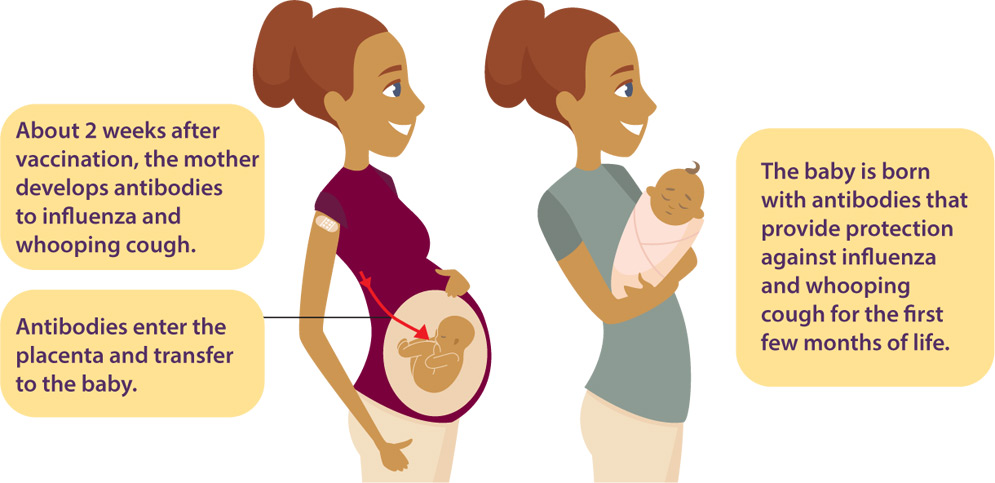
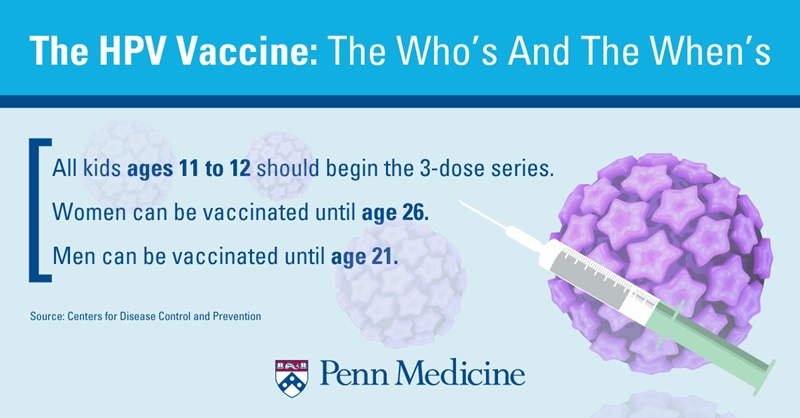
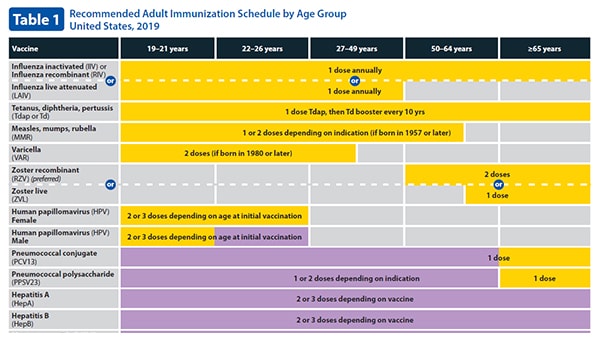
Varicella-containing vaccine is recommended for children at 18 months of age as MMRV measles-mumps-rubella-varicella vaccine. The chickenpox vaccine also protects you from getting shingles later in life.
Ismp National Vaccine Errors Reporting Program One In Three Vaccine Errors Associated With Age Related Factors Institute For Safe Medication Practices
Varicella is caused by the varicella-zoster virus VZV a form of the herpes virus.
/doctor-giving-toddler-girl-a-shot-149285208-2eb8d45de6a8477a9c9c32b0359b7a22.jpg)
What is the chickenpox vaccine abbreviation. A vaccine against chickenpox is available to protect groups who are particularly at risk of complications from the disease. Chickenpox vaccine overview - NHS. Varivax is the single-antigen varicella vaccine.
Pneumococcal Conjugate Vaccine 7-valent no longer available PCV13. Pneumococcal Polysaccharide Vaccine 23-valent formerly called PPV or PPV23 PRP. Chickenpox also called varicella is usually a mild disease that doesnt last long in children but it can be more severe particularly in adults.
A virus causes this condition. The vaccine is not currently offered to all children as part of the routine schedule in the UK. Varicella chickenpox is a very common childhood disease.
It causes a blister-like rash itching tiredness and fever. How to Pronounce Varicella. Highly purified gelatine used as a stabiliser Varivax only - there is no gelatine in Varilrix.
Chickenpox also called varicella is characterized by itchy red blisters that appear all over the body. In some countries all children are offered the MMRV vaccine a combined measles mumps rubella and varicella vaccine. Hepatitis B vaccine overview Chickenpox vaccine.
Chickenpox used to be very common in the United States. Varicella is a highly contagious virus that is spread from person-to-person through the air or by contacting the fluid from. Apart from the active ingredientsthe antigens the vaccines may contain very small amounts of these added ingredients.
Primary infection with varicella-zoster virus causes varicella chickenpox. ProQuad is a combination measles mumps rubella and varicella MMRV vaccine. Find out how the chickenpox varicella vaccine protects vulnerable people from the potentially dangerous consequences of this normally mild illness.
The chickenpox vaccine also known as the varicella vaccine Varivax Merck is a live attenuated vaccine made from the Oka strain of the varicella zoster virus. MP3 Chickenpox is a very contagious disease caused by the varicella-zoster virus VZV. It is usually mild but can be serious especially in young infants and adults.
Varivax Medication Uses How To Use Side Effects Precautions Drug Interactions Overdose Notes Missed Dose Storage. It often affects children and was so common it was. The chickenpox vaccines used in the UK are called Varilrix and Varivax.
Before the varicella vaccine Varivax was released for use in 1995 nearly all of the four million children born each year in the United States contracted chickenpox resulting in hospitalization in five of every 1000 cases and 100 deaths. Vaccination is a safe and effective way to protect you from a serious case of the disease. Live-attenuated vaccines are a very effective type of vaccine used in the prevention of diseases including influenza chickenpox measles polio and TB.
Contains a combination of measles mumps rubella and varicella chickenpox vaccines which is also called MMRV Is only licensed for use in children age 12 months through 12 years ProQuad can be given to children for their routine two doses of chickenpox vaccine at age 12 through 15 months and age 4 through 6 years. BCG tuberculosis TB vaccine overview BCG TB vaccine side effects BCG TB vaccine FAQs Who should have the BCG TB vaccine. Varicella Vaccine Effectiveness and Duration of Protection Two vaccines containing varicella virus are licensed for use in the United States.
Pneumococcal Conjugate Vaccine 13-valent replaced PCV7 PPV23 or PPV Pneumococcal Polysaccharide Vaccine 23-valent replaced by the term PPSV23 PPSV23. Chickenpox epidemiology Chickenpox immunology Chickenpox prevention control Chickenpox Vaccine administration dosage Chickenpox Vaccine immunology. Varicella virus vaccine chickenpox - injection var-i-sel-a BRAND NAMES.
Varicella is a highly contagious infection caused by varicella-zoster virus.